Keeping track of current trends in FDA medical device recalls is an integral aspect of risk management and staying informed about the FDA’s current line of thinking. This is especially vital for MedTech startups lacking regulatory experience, as tracking recall trends can inform crucial decisions.
Class 1 medical devices, falling under the FDA’s general controls, present the lowest level of risk to users. Taking a look at the class 1 recalls this far in 2023, there are four major trends observed:
Poor Design Controls
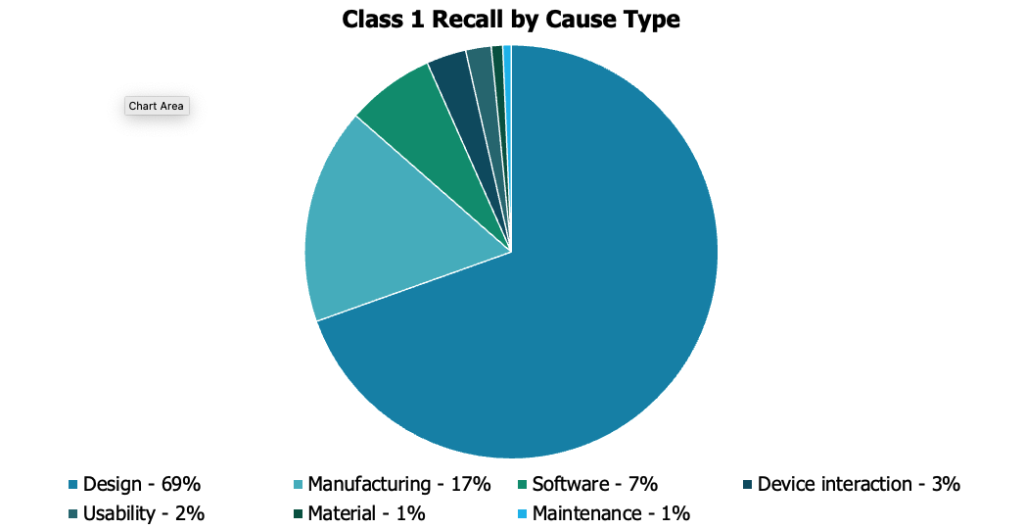
Far surpassing other causes, design-related issues account for over 60% of Class 1 recalls in 2023. Effective design controls ensure consistent, high-quality product. However, gaps in this process expose MedTech companies to design issues and regulatory risk.
Understanding how to complete risk assessments, FMEAs, and DHFs are just a small part of an effective design process.
Scrutiny on safety and performance
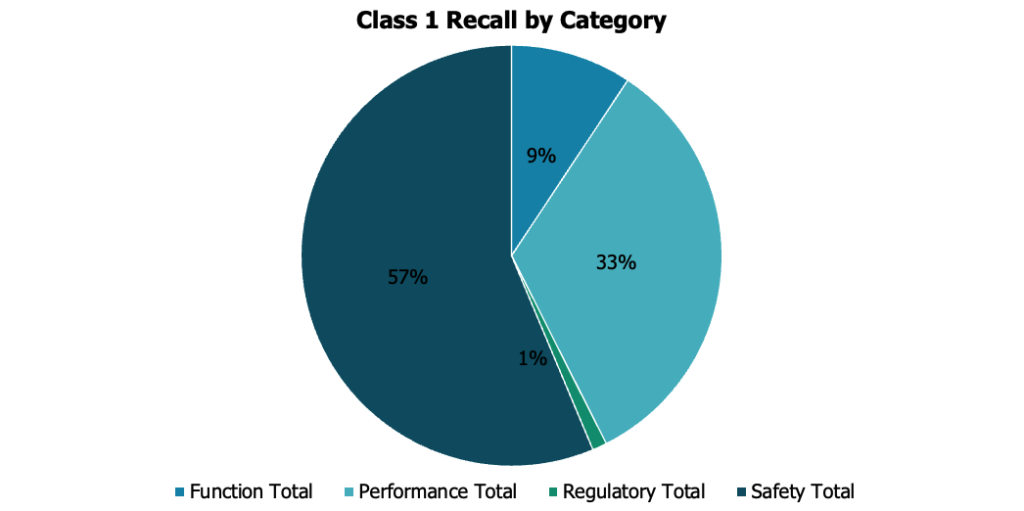
More than half of all Class 1 medical device recalls in 2023 relate to safety. Performance (33%) was the other major category, while function (9%) and regulatory (1%) were the remaining factors.
Device safety can range from potential for injuries to contamination. The lack of appropriate premarket clearance or approval can also result in severe consequences, underscoring the pivotal role that experienced personnel play in implementing stringent safety measures and ensuring regulatory compliance within the medical device industry.
Primary hazards – failure & function
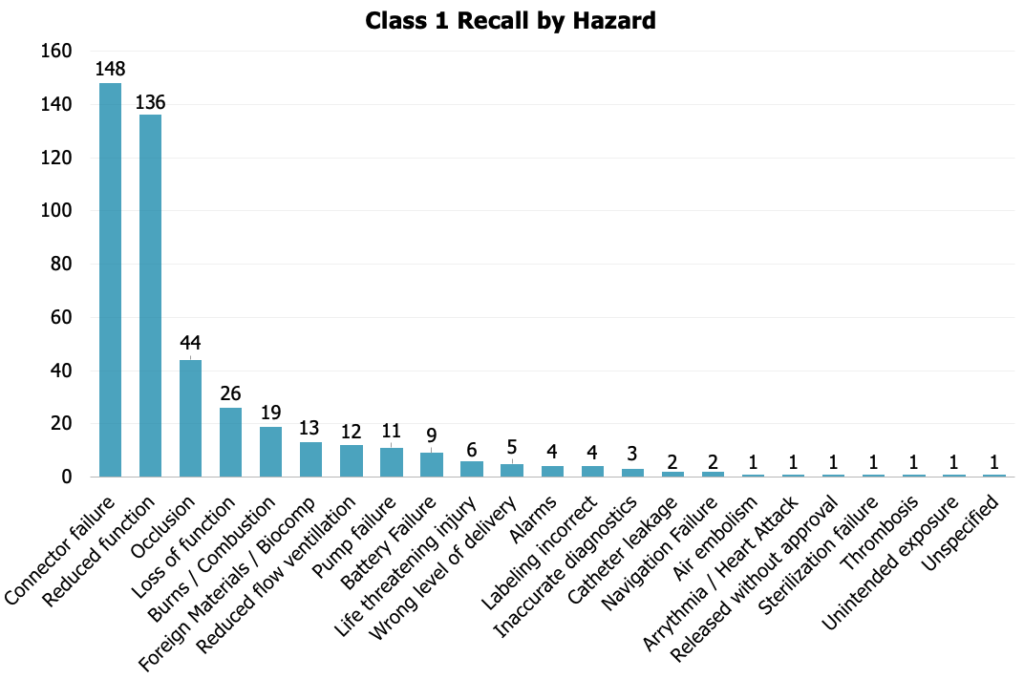
The primary hazards associated with Class 1 recalls in 2023 revolve around failures and malfunctions. Any failure or malfunction can have severe consequences. Understanding the intricacies of your device, conducting thorough testing, and implementing robust quality control measures become imperative in preventing failures and malfunctions.
MedTech companies need to invest in comprehensive testing procedures and collaborate closely with regulatory bodies to ensure the highest standards of safety and functionality are met.
Main devices – defibrillators & endotracheal tubes
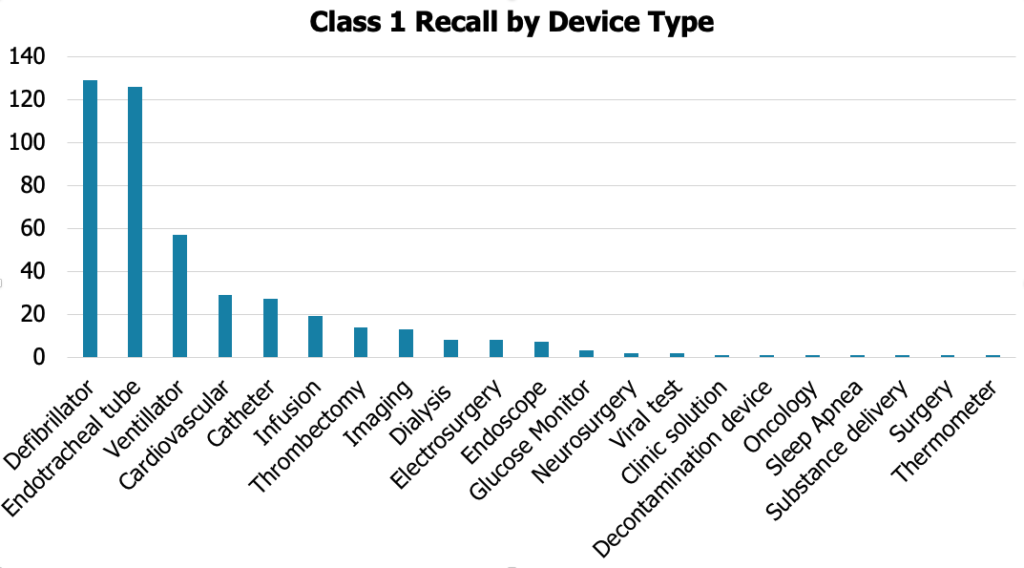
Defibrillators and endotracheal tubes are the focal points of Class 1 recalls in 2023. The high rate of recalls in these device types underscores the importance of addressing design flaws and manufacturing inconsistencies promptly. Issues like material defects or inadequate manufacturing processes can compromise the functionality of medical devices, posing a direct threat to patient safety.
MedTech companies manufacturing medical devices must conduct rigorous quality assessments, adhere to stringent design controls, and stay vigilant in monitoring their products’ performance post-market. Collaborating with healthcare professionals and regulatory agencies is vital to swiftly address and rectify any issues related to these critical medical devices.
Conclusion
The FDA’s focus on design controls, safety and performance, particularly in devices like defibrillators and endotracheal tubes, highlights the critical nature experienced personnel play in addressing emerging challenges promptly and upholding the highest standards of patient safety.
Staying informed about these trends not only aids in risk management but also serves as a guide for MedTech startups navigating the complex regulatory landscape. By learning from the trends of the past, the industry can collectively move towards a future where medical devices are not only innovative but also consistently reliable and safe.
If you would like to discuss design strategy with an experienced quality professional, our experienced team is ready to work with you. Contact us at quality@cannonqg.com.